Perspectives
Navigating the Ever-Evolving World of COVID-19 Testing
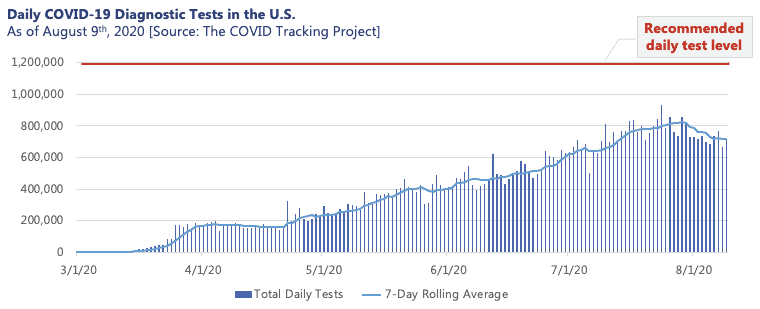
Over half a year into the pandemic, many of us have personally experienced or know someone who has faced the quandary that is the COVID-19 testing landscape. While the policies and obstacles encountered by those seeking a test vary meaningfully by geography, there is no question that rigorous testing has and will continue to play a critical role in the monitoring and containment of the COVID-19 pandemic. For states such as New York, which implemented rigorous testing procedures early on, testing proved pivotal in successfully flattening the local curve throughout the early months of the pandemic. However, the complications of the current testing environment have left consumers, employers, and even state and local governments with shaky guidance on navigating an increasingly complex and strained system. With this, the U.S. remains short of the 1.2M tests per day recommended by public health experts for mitigation of the virus.
What are COVID-19 tests and who should get tested?
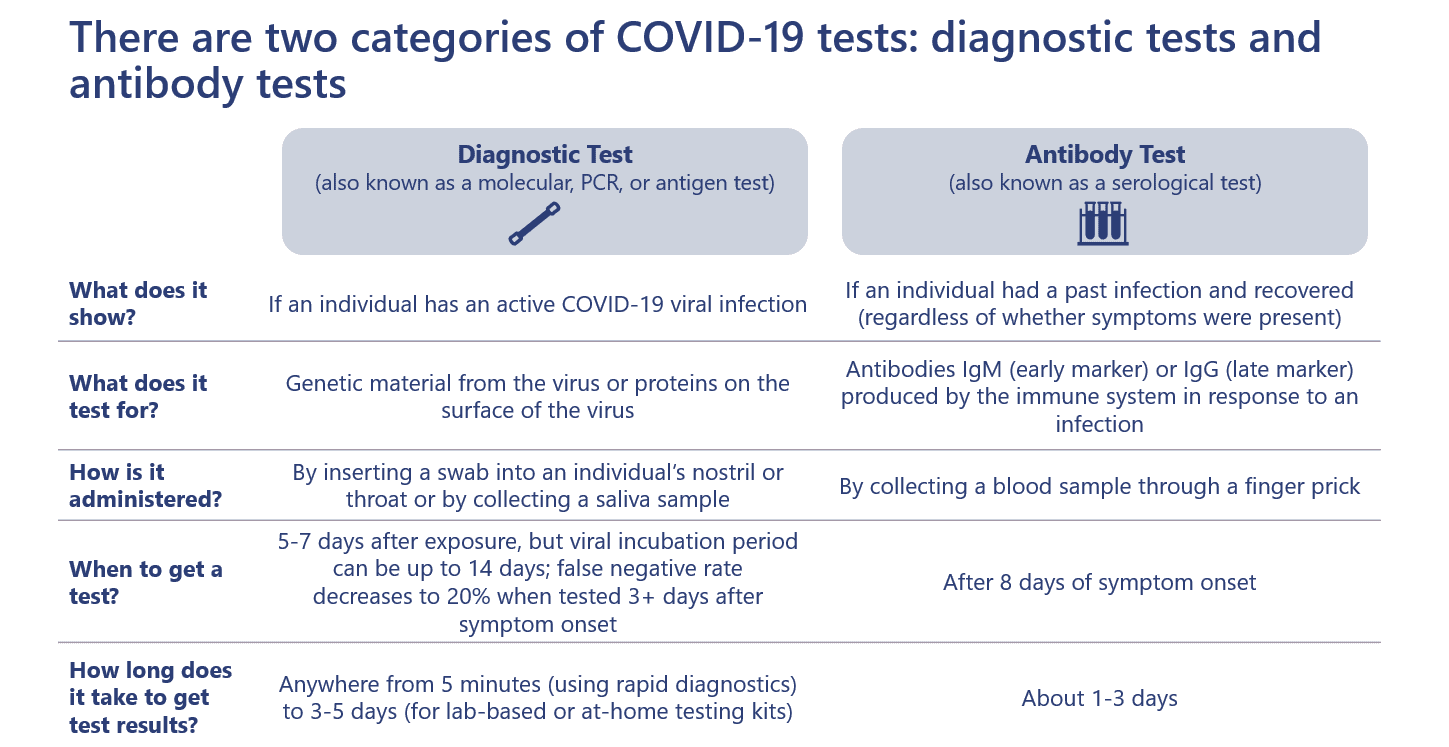
There are two types of tests available for consumers with different purposes. The diagnostic test (also known as a molecular, PCR, or antigen test) detects if an individual has an active COVID-19 viral infection. The antibody test (also known as a serological test) detects whether an individual had a past infection and developed an immune response.
Current CDC guidelines recommend that individuals should get a diagnostic test if they:
- Experience COVID-19 symptoms
- Have had close contact with a confirmed or suspected case
- Live or work with a vulnerable population (e.g., prisons, long-term care facilities)
- Participate in a contact tracing campaign
The antibody test is recommended for individuals who:
- Were previously infected with the COVID-19 virus and
- Are 1-3 weeks beyond initial symptom onset and
- Are directed by their physician to have the test as part of their clinical assessment or are part of a population immunity study
Timing is an important consideration: consumers should wait 5-7 days after suspected exposure, but no more than 14 days after symptom onset, before receiving a diagnostic test to avoid false negatives. Similarly, antibody tests should not be administered earlier than 1 week after symptom onset or 3 weeks after exposure.
However, accessing either test can be challenging as some testing locations are plagued with delays and inconsistencies. Diagnostic testing requirements in particular are rapidly changing as supply chains are strained and vary significantly across states and testing sites. With this, it is important that consumers do their state-specific research to identify whether they qualify for testing, where to access testing, and whether a doctor’s order is required. All cost sharing for both tests is waived across public and private insurers for providers that directly bill insurance.
Which tests are best?
Accuracy of both diagnostic and antibody tests vary meaningfully. In general, healthcare providers have stopped using diagnostic tests with questionable accuracy, though the Abbott ID NOW rapid test remains available despite several adverse event reports claiming false negatives. Diagnostic tests manufactured by the CDC, Cepheid, and Roche have been shown to have the highest accuracy (>95%). Some testing providers have elected to use a self-administration diagnostic technique to improve efficiency and protect healthcare workers – initial studies show that this technique is just as accurate, although more research is needed.
As accuracy and cost continuing top priorities for manufacturers, Abbott announced the launch of a new and more accurate (>97%) rapid response diagnostic, BinaxNOW, which will be made available for $5. The test will deliver results in 15 minutes with no instrumentation. Abbott intends to ship tens of millions of tests in September 2020 and ramp up to 50 million tests by October.
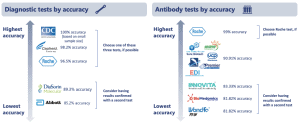
Antibody tests are generally less accurate than diagnostic tests, though this is improving with new FDA authorization requirements. The Roche test has the highest accuracy at 99% (that is, only 1% will produce a false negative), while several other manufacturers have lower accuracy (<91%). Still, antibody tests have a very low false positive rate (<5%), meaning that such antibody tests are unlikely to falsely report the presence of a past infection.
Home diagnostic testing offers a more convenient option for some consumers. While accuracy data is still limited based on small sample sizes, home delivered tests provide several benefits including reduced exposure, preservation of PPE, and a less invasive, saliva-based collection technique. There are several providers of at-home testing kits; however, availability of at-home tests varies by state. Of note, some of the at-home testing providers require that the consumer pay an upfront out-of-pocket cost that can be submitted to insurance for full or partial reimbursement.
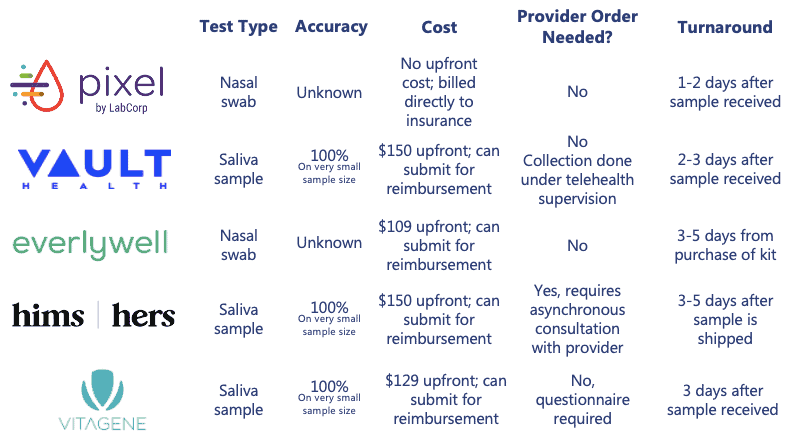
Across the three testing types and modalities, it is important to understand that not all tests are created equal, and accuracy data remains limited. Consumers should research test availability, as accuracy and price can vary significantly. For diagnostic tests in particular, rapid tests may be convenient, but are often not as accurate as lab-based diagnostic tests (i.e., sometimes only 70% accurate).
How should results be interpreted?
A positive diagnostic test result is straightforward and requires following CDC guidelines to avoid transmission. However, a positive antibody result is not as clear cut. A positive antibody test can indicate antibodies from an infection with the SARS-CoV-2 virus, or from another virus in the coronavirus family; thus, a positive result does not necessarily indicate a previous COVID-19 infection.
Further, it remains unclear how much protection such antibodies provide and how this potential protection changes over time. The most recent data on antibody tests indicates that antibodies may only last two to three months, particularly in asymptomatic individuals. However, other types of antibodies (called “neutralizing antibodies”) may remain present, and immunity-driving T-cells and B-cells can also re-activate an immune response upon re-infection. Those who test positive for antibodies should therefore not consider this an “immunity certificate” as several major unknowns remain.
Nonetheless, this insight about antibodies does not mean that COVID-19 vaccine prospects are doomed. In fact, the immunity created by a vaccine differs significantly from the immunity created by a natural infection. Vaccines are intelligently designed with antigens that focus the body’s immune response on the weak portions of the virus. Additionally, the use of an adjuvant in a vaccine (the ingredient that potentiates the immune response) serves to boost and lengthen the immune response when compared to the antigen alone. More information is still needed to determine the best path and timeline to a vaccine.
What is the role of key healthcare stakeholders in improving the testing landscape?
Payers have the unique capability to encourage testing across their members by offering lasting coverage, establishing partnerships, and disseminating accurate information and education. Payers also have the opportunity to promote the use of the most accurate tests and expand coverage to include at-home testing providers upon the release of accuracy data. To promote population-wide utilization, payers should continue to waive cost-sharing for testing at a set frequency with flexibility based on exposure risk.
Additionally, employers will need to develop robust testing strategies, but their success will be contingent on test availability in each company’s geographic location. Employers should establish partnerships with diagnostic testing providers, with careful consideration of accuracy data. In tandem, testing and contact tracing policies and procedures should be established to enable a safe return to work.
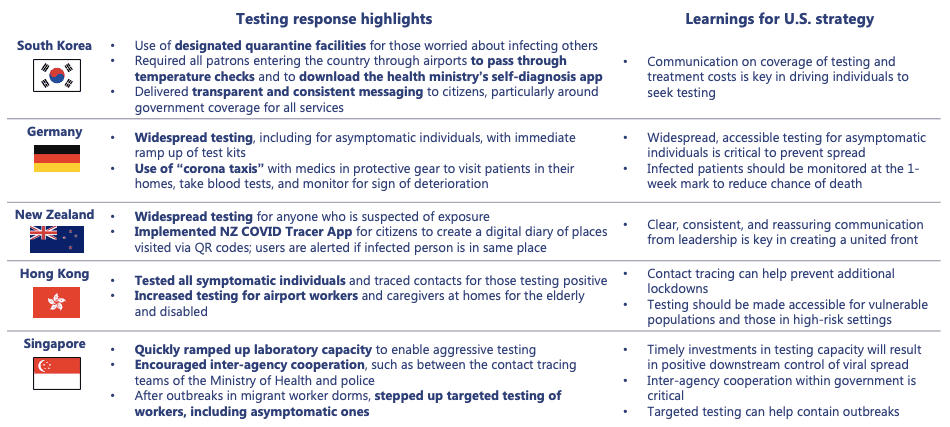
From a public stakeholder perspective, in the absence of a national testing strategy, state and local governments can coordinate to share resources. States should establish coordination entities to assist labs at capacity with identifying idle laboratory resources. Coordination should include efficient distribution of resources to minimize state-to-state competition. As more data is collected, state and local governments must provide guidance to providers, employers, and payers on the availability of resources and the most accurate testing options. Public stakeholders should not reinvent the wheel – they should leverage global best practices that have proven effective to improve upon the U.S. diagnostic testing strategy.
7wireVentures predictions for the testing market
 Testing will increasingly shift from predominantly symptom-based and exposure-based to monitoring and precautionary testing
Testing will increasingly shift from predominantly symptom-based and exposure-based to monitoring and precautionary testing
Employers, schools, and healthcare organizations will integrate regular testing into their operating procedures. Some employers will require that employees receive a negative test result before returning to work, and retailers such as CVS Health will help make this possible through return to work services. Schools and universities may also require that students receive a negative test result before returning to campus. Further, testing will continue to serve as a required prerequisite for elective procedures.
 Competition between organizations to build testing capacity will grow, causing additional harm to the most vulnerable
Competition between organizations to build testing capacity will grow, causing additional harm to the most vulnerable
With employers, schools, and healthcare organizations looking to purchase large quantities of tests to accommodate a return to business operations, the shortage of testing supplies and services is likely to continue. As private organizations race to establish testing capacity for frequent testing of employees, vulnerable populations without access to such channels will be left with limited access. Government support will be needed to ensure adequate lab capacity for state-run testing sites such as those located in homeless shelters.
 Advancements in research will drive testing and analysis into consumers’ homes
Advancements in research will drive testing and analysis into consumers’ homes
Centralized collection and processing for diagnostic tests is a costly and inefficient process today, and has been a major source of delays. As testing technology advances, both collection and processing of test specimens will shift from testing centers and centralized labs to consumer homes to enable convenient testing at scale. With this shift towards home testing, rigorous reporting through the use of digital solutions will be imperative to ensure accuracy of public health information.
We wish all readers continued health and hope that your COVID-19 testing needs are met quickly, accurately, and without cause for concern.